Dr. Michael Erb, Dr. Ranganatha Sitaram
About the authors of this article
Michael Erb (michael.erb@med.uni-tuebingen.de) studied physics at the Universities of Karlsruhe and Tübingen, Germany, and graduated in 1985. He pursued his Ph.D. Studies on artificial neural networks at the Max Planck Institute for Biological Cybernetic in Tübingen from 1986–1990. After a research visit at the Institute for Brain Research, University of Düsseldorf, he was a research fellow at the Institute for Neurophysics, University of Marburg, Germany. Since 1995, he has been a research fellow at the Department of Neuroradiology, University of Tübingen, performing fMRI studies on a variety of research topics. He is involved in building MR compatible stimulation devices and programming MR pulse sequences and analysis software.
Sitaram Ranganatha (sitaram.ranganatha@uni-tuebingen.de) obtained his bachelor and master degrees in engineering from the University of Mysore and Bharathiar University in India in 1990. Until 1992 he worked as a senior research fellow in the Bhabha Atomic Research Center, India, pursuing a project aimed at developing adaptive robotic systems. From 1992 to 2004 filled various positions such as a senior engineer and leading scientist, in the Kent Ridge Digital Labs and the Institute of Infocomm Research of Singapore. He worked in a variety of research fields including artificial intelligence and knowledge systems, intelligent transport systems, cellular communication, smart devices, synthetic creatures and brain-computer interfaces. Since 2004, he has a full-time position as a research scientist and faculty member at the Institute of Medical Psychology and Behavioral Neurobiology, Tuebingen, headed by Prof. Dr. Niels Birbaumer. He is mainly involved in developing brain-computer interfaces based on near infrared spectroscopy and real-time functional magnetic resonance imaging as tools for communication and rehabilitation of patients with paralysis, and emotional and motor disorders. He obtained his PhD in neuroscience in 2008 for his work on metabolic brain-computer interfaces with a summa cum laude. He is one of the recipient’s of the President of India’s award for nonresident Indian scientists to collaborate with Indian universities, based on which he is now a visiting professor at the Sri Chitra Tirunal Institute of Medical Sciences and Technology in Trivandrum, India. His scientific interests lie in neural learning and plasticity, scientific study of consciousness and the development of advanced neuroimaging methods. He has authored seven international patents, and numerous engineering and scientific publications. He lives with his wife and two children in Tuebingen.

Dr. Sitaram (left) and Dr. Erb
1 Neuroscience and Meditation
Since the early sixties of the last century, neuroscientific investigations of meditation have been performed with electroencephalographic (EEG) recordings. Although neuroelectric correlates of altered states of consciousness during meditation are not yet firmly established, the primary findings have implied increases in theta and alpha band power, and decreases in overall frequency (for a review see Cahn & Polich 2006). With the development of neuroimaging techniques like Positron Emission Tomography (PET) and functional Magnetic Resonance Imaging (fMRI) in the 80s and 90s, these new methods have also been applied to reveal the neurophysiologic correlates of modified self-experience in meditation practice.
For many years the 14th Dalai Lama Tendzin Gyatsho has been interested in Western science. In 1987, a series of meetings—the Mind and Life conferences—were initiated between the Dalai Lama and a number of prominent neuroscientists. This led to numerous neuroscience studies on meditation (Barinaga 2003) including neuroimaging, especially at the University of Wisconsin (Madison) surrounding the group of neuroscientist Richard Davidson.
Unlike Western science, Buddhist philosophy assumes the existence of six senses that allow the mind to interpret the world. In addition to the senses of seeing, hearing, touch (body sense), taste and smell, the sixth sense or inner sense allows us to monitor our thoughts and feelings. Buddhism is therefore viewed as a science of the mind with insights based on more than 2,500 years of studying the mind by introspection. Western neuroscience may thus gain valuable insights from this experience by adapting some of these practices in theoretical and experimental investigations.
A fundamental question for neuroscience is the elucidation of the relationship between subjective experience and neural firing. A flash of light, for example, produces measurable evoked potentials in the visual cortex. While it is relatively straightforward to relate simple sensory stimuli to brain activity, it is, however, far less obvious how complex subjective states such as the experience of meditation are reflected in brain activity. Experimental approaches to meditation can be broadly classified into two major types with different goals concerning the states and traits of meditation (Cahn & Polich 2006). The first one investigates the differences between the mental state in normal day-to-day thinking, and the specific mental state during an ongoing meditation session. The second approach investigates the effect of meditation that persists even when not presently engaged in meditation practice. Both approaches have provided insight into the effects of meditation practice on the mind and brain of humans.
2 The Tübingen Experiment on Śūnyatā meditation
In 2006, the Śūnyatā meditation center in Stuttgart (Germany) contacted Dr. Michael Erb and asked if he would be interested in conducting neuroimaging experiments on brain activity of the Buddhist master of the school, Master Thích Thông Triệt, during meditation in the MR scanner. After discussing the challenges and scope of such an investigation and consulting with another neuroscientist, Dr. Ranganatha Sitaram, the authors consented to embark on a series of experiments to examine Śūnyatā meditation with different levels of expertise. The aim of our study was to investigate whether there are differences in brain activations between meditation and normal day-to-day thinking. And if so, we wanted to further identify brain activations pertaining to different stages and techniques of Śūnyatā meditation. The idea was to investigate whether specific brain activity is related to different techniques of meditation.
Before discussing in more detail our hypotheses, an explanation of the central doctrine of Śūnyatā meditation is useful for a better understanding of the framework. Buddha described the methods of meditation as follows (as per Bāhiya Sutta, Anderson 2002):
“Please train yourselves thus: In the seen, there will be just the seen. In the heard, there will be just the heard. In the sensed, there will be just the sensed. In the cognized, there will be just the cognized. When for you, in the seen there is just the seen, in the heard just the heard, in the sensed just the sensed, in the cognized just the cognized, then you will not identify with the seen, and so on. And if you do not identify with them, you will not be located in them; if you are not located in them, there will be no here, no there, or in-between. And this will be the end of suffering.”
The word Śūnyatā means emptiness in Sanskrit. This meditation practice has its origin in the Buddhist philosophy that signifies the impermanent nature of form, or, in other words, that objects in the world do not possess essential or enduring properties. In Buddhist spiritual teaching, cultivating insight into emptiness leads to wisdom and inner peace. Śūnyatā meditation practice is aimed at developing an ability to avoid discursive (wandering, longwinded) thought. Instead insight into the nature of reality is acquired through direct perception of the internal (bodily) and external (sensory) states. So, automatic associations of former episodes from memory, evaluation of perceptions with respect to ones’ own existence and planning of future actions will be reduced, whereas self-awareness and awareness of the things in the world will increase.
2.1 Hypothesis
The aim of the present study was to investigate state changes in the brain and accompanying bodily reactions during Śūnyatā meditation, when confronted with a variety of external stimuli. Based on the rationale behind the Śūnyatā practice, we hypothesized that the following state changes occur during Śūnyatā meditation in comparison to normal day-to-day thinking: First, brain regions that have been associated with memory retrieval, planning and executive control will be deactivated; second, brain areas related to interoception and sensory perception will be activated; and third, the respiration rate (and possibly other physiological signals) will be reduced.
As mentioned in the chapter on Biofeedback in Zen Meditation (Chapter III, Article 4), the Buddhist doctrine is embodied in a practice of meditation that guides the practitioner to “Not Naming the Object” and rather helps him to “see it as seeing” (bare or natural seeing), “hear it as hearing” (bare or natural hearing), “feel it as feeling” (bare or natural touch) and “know it as knowing” (bare or natural cognition).
We investigated the following four different meditative practices: natural seeing, natural hearing, natural touch and natural cognition. The objectives of the study were to identify brain regions associated with the different sensory states of meditation. In addition, we wanted to investigate whether there were common activations across all these practices. This was motivated by the idea that all practices are based on the Buddhist doctrine explained above. We expected to find activations in visual areas (occipital cortex) during natural seeing, in auditory areas (temporal lobe) during natural hearing and somatosensory areas (postcentral gyrus) during natural touch. We also expected brain activations common to all meditation practices in the temporo-parieto-occipital junction (e.g. Brodmann Area (BA) 39), which plays a role in multi-sensory integration (Fig. 1).
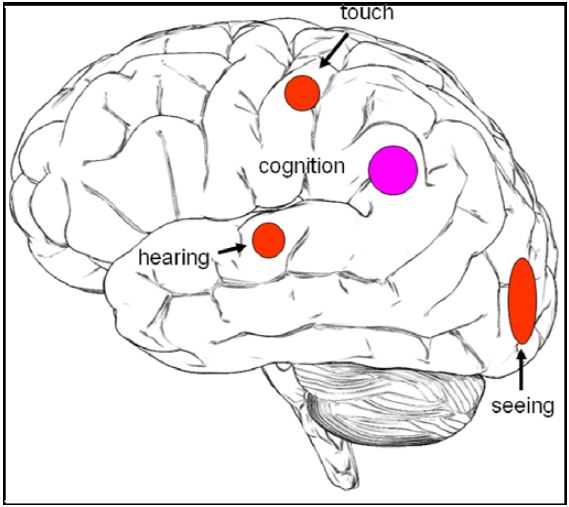
Figure 1: Hypothesis
Since 2006, we have scanned several meditators from the Śūnyatā Meditation Stuttgart e.V. (Germany) and monks and nuns from Śūnyatā Meditation Center in Riverside (CA, USA); a total of 8 participants in 18 sessions, including 6 sessions with Master Thích Thông Triệt.
In this chapter, we will present the findings from the group and a few single cases and then focus on the results of the experiments with the Master. It should be noted here that while the method allows a description of brain activations elicited during meditation, it is not suitable to investigate the effects of meditation on the autonomic nervous system and physical health.
2.2 Measurement Methods
For a better understanding and interpretation of the results of our experiments, it is necessary to provide a summary of the capabilities and limitations of the neuroimaging approach used in this study.
2.2.1 Functional magnetic resonance imaging (fMRI)
Functional magnetic resonance imaging (fMRI) is currently one of the most widely used methods for mapping human brain functions. fMRI measures the hemodynamic response to neural activity in the brain. This is possible because increased activity in nerve cells also increases their consumption of oxygen. The local response to this oxygen utilization is to increase blood flow to regions of increased neural activity, which occurs after a delay of approximately 1–5 seconds. This hemodynamic response rises to its peak of intensity around 4–5 seconds, before falling back to baseline (and typically undershooting slightly). Thus, neural activity leads to local alterations in the relative concentration of oxygenated hemoglobin and deoxygenated hemoglobin, cerebral blood volume and blood flow. As hemoglobin is diamagnetic when oxygenated and paramagnetic when deoxygenated, this difference can be measured by magnetic susceptibility sensitive MR sequences (e.g. echo planar imaging – EPI). The resulting blood-oxygenation-level dependent signal (BOLD signal, Ogawa 1990) is an indirect measure of the corresponding neural activity. In a typical fMRI experiment, the BOLD signal differences can reach up to 5% of its baseline value, implying that these small effects can only be detected by many fMRI measurement volumes and sophisticated statistical analysis methods. The standard design of fMRI experiments is the so-called block design, where blocks with stimulation or task condition are alternated with blocks of rest or control condition. As the length of the hemodynamic response function (HRF) is about 15 seconds, conditions typically alter every 20 to 30 seconds to be able to obtain multiple rises and falls of the response. With a typical spatial resolution of 3 mm, it is possible to scan the whole brain with EPI sequences in about 2-3 seconds, so that as many as 10 brain volumes can be acquired within each block. After averaging the measured volumes in all rest blocks and all task blocks respectively, one can determine the difference between these mean values to show locations with higher values in task blocks above a selected threshold as overlays on anatomical images, typically measured with a resolution of 1x1x1 mm3. A preliminary online analysis can be performed immediately on the scanner computer during fMRI acquisition of the experimental protocol. For refined analysis, there are several statistical methods available such as modeling the expected hemodynamic response and estimating parameters of a general linear model with the measured data. In addition some preprocessing steps like head motion correction, that is realigning the images to the first volume, and smoothing of the images can help to improve the statistical power of analysis.
Experimental protocol
We were confronted with two potential problems in the experimental setup. One concerns the scanner environment which is not optimal for meditation: the participants are placed in a narrow scanner with considerable measurement noise. To overcome this issue, it was important to have highly experienced meditators who would be able to adjust to the situation. The other problem is related to setting up
suitable task and control conditions. For the present experiment, it was doubted whether meditators could achieve rapid switching between normal thinking (control condition) and meditation (task condition) within a few seconds. It was therefore decided to use longer block lengths of alternating baseline (3 times of 2 minutes each) and meditation (2 times of 3 minutes each) conditions (Fig. 2). This was a reasonable trade-off between the requirements of effective fMRI measurements and normal duration of meditation sessions.
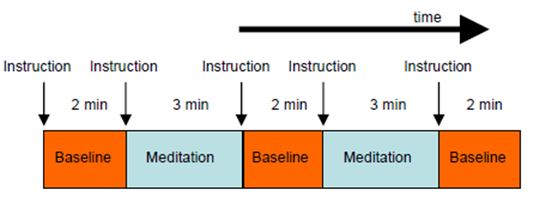
Figure 2: Experimental design
2.2.2 Physiological Signals
In addition to the BOLD signal, we recorded respiration and pulse signals to calculate time courses of respiration amplitude and frequency together with heart beat frequency. From these signals, we tried to estimate the time course of actual meditative states. There was no clear indication or previous data from Śūnyatā meditation suggesting that respiration rate changed as a consequence of the meditation state, or whether control of respiration rate was used to reach the meditation state as in other meditation practices. After each session, participants were asked to rate the depth of the meditative state achieved in each meditation block by using a questionnaire.
2.2.3 Electroencephalography (EEG)
For some sessions, we were able to simultaneously recordEEG signals from 31 electrodes with a MR compatible EEG amplifier and EEG cap (BrainAmp MR, Brain Products GmbH, Munich, Germany). EEG measures the brain’s electrical activity directly, while fMRI records changes in blood flow. Combining EEG and fMRI allows for brain signals to be recorded at a high temporal as well as spatial resolution. Lutz and colleagues (Lutz et al. 2004) have shown increased gamma oscillations during meditation. We thus expected to classify different stages of meditation with the help of EEG signals and use these time courses to find corresponding locations in the fMRI signal. It should be noted here that there are still technical difficulties associated with combining fMRI and EEG measurement techniques, including the need to remove the MRI gradient artifacts present during MRI acquisition and the cardioballistic artifact (resulting from the pulsative motion of blood and tissue) from the EEG signals. These difficulties may interfere with data interpretation.
2.3 Additional tasks
In addition to the meditation protocol described previously, we performed an extended examination with the Master Thích Thông Triệt with paradigms targeting object recognition and language related areas, different levels of thinking, and the differences between sensory stimulation and no stimulation under normal thinking and meditation conditions. In these sessions, we used a block design with
blocks of 30 seconds each for more efficient fMRI measurement. A further set of sessions tested four different levels of awareness, namely, “verbal”, “tacit”, “awakening” and “cognitive” awareness.
2.3.1 Visual and auditory naming of animals and tools
In the visual naming task, we projected pictures of animals and tools onto a screen inside the MR scanner that was visible to the participant via a mirror. The participant was instructed to name the object using inner speech (without actual vocalization). As we were only interested in regions engaged in object recognition and naming and not primary visual processing, we showed stimuli of scrambled images as control condition.
In the auditory naming task, we presented short sounds from animals and tools to the participant with MR compatible headphones. Each sound had a duration of 2.5 seconds and was presented randomly two times in the experimental session. To activate the primary auditory regions in a comparable way, we used the same sounds but scrambled in the control periods. Control and task periods were signaled to the participant with a red or green rectangle, respectively. With these tasks, we wanted to identify brain areas for object recognition and language.
Visual naming should activate the higher level of the ventral visual stream including the fusiform gyrus (BA37, bilateral) engaged in visual object recognition, as well as the language areas for generating the corresponding nouns (Wernicke’s area, BA22, BA39, BA40, left) and performing inner speech (Broca’s area, BA44/45, premotor area, BA6, left). Auditory naming should activate the superior temporal gyrus and again the language and speech areas. Hence, with this approach, we expected to identify the brain regions where we expected changes in different meditation methods.
2.3.2 Different levels of thinking
To distinguish between different levels of thinking, we conducted a series of sessions with the Master. The protocol comprised 13 blocks with different thinking tasks (duration = 30 seconds), namely “intellect”, “mind-base”, and “consciousness” in alternating order. These terms were displayed as written words on the screen for the corresponding period of 30 seconds each and were used as a trigger for the participant to invoke “thinking.” “Intellect” corresponded to cognitive thinking and reasoning, whereas the “mind-base” condition involved relaxed playing around with thoughts (“inner chatter”) and “consciousness” referred to being aware of the self. “Counting” was used as a reference task as this can be done more or less automatically.
2.3.3 Different levels of meditative depth (awareness)
In an additional series of sessions with the Master, four different levels of meditation depth as per Buddha’s description were investigated: “verbal awareness”1, “tacit awareness”2, “awakening awareness”3 and “cognitive awareness”4. These measurements were also performed in a block design of 2 minutes of baseline (3 times) and 3 minutes of meditation (2 times), as much faster switching between the levels could have been difficult. Master Thích Thông Triệt characterized these four states in the following way:
- Verbal Awareness is equivalent to the first level of Samādhi, or Savitakka Avicāra Samādhi, in the original teachings.
The inner silent dialogue, or vicāra, refers to the mental images that arise from the memory during the sitting meditation. It hinders the practice of meditation. To prevent the inner silent dialogue from surfacing to consciousness, the Buddha taught “silent thinking”, or avicāra, a meditation technique characterized by silently thinking the phrase, “When I breath in, I know I am breathing in; when I breath out, I know I am breathing out”. This technique quiets the inner dialogue by focusing the mind on the task of breathing. The practitioner then experiences the state of “Vitakka without vicāra” Samādhi, or verbal thinking but noninner-silent-dialogue Samādhi.
- Tacit awareness, Avitakka Avicāra Samādhi, similar to the second level of Samādhi, meaning “wordless thinking and non-discursive dialogue”.
Tacit awareness means wordless or non-verbal awareness. At this state of awareness, the practitioner masters the chattering mind during four common daily activities: walking, standing, lying, and sitting. By quieting the mind, the practitioner inactivates the networks of perception, one component of the Five Aggregates which, according to the original meditation and Zen sect, is the most important Samādhi. Through this level of Samādhi, the practitioner is able to attain all the other levels of meditation, such as Śūnyatā Samādhi, Formlessness Samādhi, or Wishlessness Samādhi. This is in contrast to the tradition of The Elders (Sthaviravadin) and Sarvastivadah. The Elders commended mind concentration (Citta ekkaggatā)
- Awakening Awareness is equivalent to the third level of Samādhi, Sati-Sampajañña, and is defined as the “full awareness or clearly comprehensive awareness without attachment to the objectives”.
Awakening awareness is different from Wakeful Awareness which is characterized by the association of the Objective with Consciousness. Awakening Awareness is a state of the mind without the involvement of the subjective, yet the presence of the Awareness only. The Buddhist Developers assumed the term “true self” or “pure self” in which the practitioner attains Samādhi in all four daily activities.
- Cognition Awareness is equivalent to the fourth level of Samādhi.
At this stage, the mind is so tranquil that the delicate breathing ceases from time to time. The Buddha calls it the “three immobile or unshakeable formations” which refers to the (1) standstill of the discursive thinking in which neither Vitakka nor Vicāra arises, (2) the standstill of thoughts in which neither feeling nor perception arises, and (3) the standstill of the body, where the breathing stops occasionally. In fact, when the practitioner reaches this stage of meditation, he can go in or out of Samādhi at ease. In Theravada Sutras, the Buddha described this status of Samādhi as “finger snapping Samādhi”. The sixth Patriarch, Hui Neng, called it the “Samādhi needless to be in or out.”
2.3.4 Different meditation tasks
As already mentioned at the beginning of this chapter, we utilized four different methods of meditation, namely natural seeing, natural hearing, natural touch and natural cognition. We used control conditions very similar to the meditation tasks to ensure sensitivity to the effects of specific meditation practices. For the condition “seeing” one picture was displayed on the screen in the control block (baseline, 2 minutes), when the participant focused attention on the image and analyzed its content (normal seeing). The same picture was used in the following meditation block (3 minutes) with natural seeing. Hence, the external input (stimulation) was exactly the same in both conditions, the only difference being the mode of processing of the input. In the same way, we played identical music continuously over the MR compatible headphones in the baseline condition and in the “natural hearing” condition. The third condition “natural touch” consisted of brush strokes that were applied every second to the right palm of the hand of the meditator. Short beeps were used to pace the speed of the strokes. The fourth condition “natural cognition” was without any sensory stimulation. The start of a new block was signaled by auditory instructions and visually by the words “Baseline” and “Meditation”, respectively, in each run.
In order to test the different activations caused by the sensory input, we conducted a series of measurement sessions in which the external input (seeing, hearing, touch) was switched on and off every 30 seconds. These sessions were repeated in the day-to-day thinking and meditation condition.
2.4 Data analysis
Analysis of the fMRI data incorporated several steps. After transferring the data to the local computer network and converting them to a file format usable for the analysis program package, the three dimensional volumes of each measurement time point were realigned to the first volume of the session to compensate for head movement over the whole measurement period. This is very important because the subsequent analysis is calculated for each volume element (voxel) separately. Therefore, corresponding brain locations have to be in the same position for the whole run. Unfortunately, this method can only correct replacements between volumes and not distortions caused by fast movements in the time period of the data acquisition.
Therefore, in the case of such fast movements, there are still remarkable signal variations in the data after movement correction. (This is the reason, why it is so important to fix the head with a foam cushion inside the scanner while the fMRI measurement is running.) In the case of group studies, it is necessary to transform the individual’s brain images into a standard coordinate system to ensure that corresponding brain regions of the individual participants are in the same position in the new datasets. One then can localize specific positions of activations in computerized brain atlases and databases to locate the precise anatomical region, and to compare with findings from other experiments.
The standard data analysis programs used in analyzing fMRI data typically estimate a general linear model (GLM) to the measured signal time courses of small brain volume elements (voxels) in order to ascertain which parts of the brain were activated in the given task. The problem with this kind of analysis is that the time course of task performance has to be known. In our case, we could only use the time course defined by our instructions. To also obtain an objective measure for the meditative state, we used peripheral physiological signals and EEG signals. The time course of the meditative state estimated from the time courses of these different signals could then be used to search for the corresponding time courses in fMRI signals. This, however, is only possible if there is a direct and constant relationship between the signals and the meditative state, which is by itself an unresolved topic of research (e.g. Lutz et al. 2004).
In a second approach, we used a data driven method, the so-called independent component analysis (ICA, see Calhoun et al. 2009 for a review), which separates from the mixed signal time-course different spatial patterns of activations which are statistically independent of each other and hence may have originated in different sources. From these automatically generated patterns, we had to sort out components with a time course related to the task. This method allows identification of constant activations over the whole meditation period as well as transient time courses. Other components related to movement or measurement artifacts may be used to correct the data.
2.5 Results
2.5.1 Analysis of the Physiological Signals
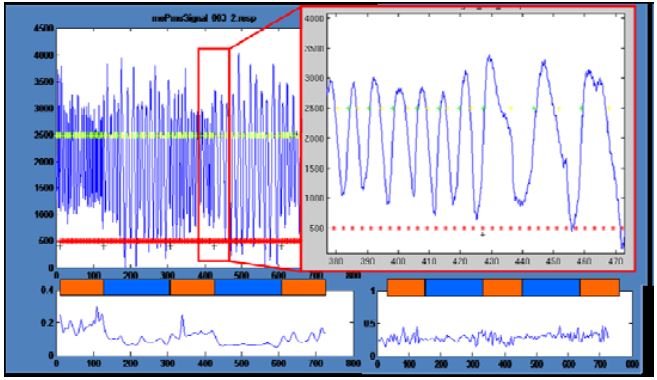
Figure 3: Physiological signals: Decrease of respiration rate
The analysis of the physiological signals revealed some interesting effects. In one participant, the time course of respiration showed a clear reduction in frequency and an increase in amplitude in the meditation periods compared to normal thinking periods (Fig. 3). Unfortunately, all other participants did not show this effect. Still another participant showed a reduction in variations in the meditation period but no changes in amplitude or mean frequency (Fig. 3 right bottom).
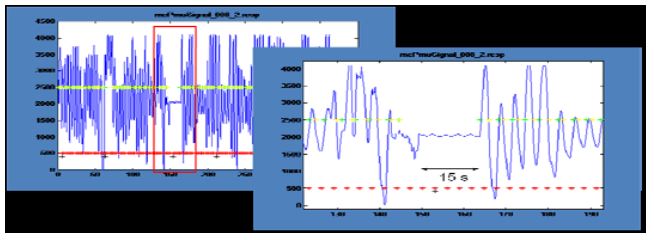
Figure 4: Physiological signals: Interruption of respiration
In a session in which the Master performed natural cognition meditation (Fig. 4), respiration was interrupted for about 15 seconds at the end of the first meditation block. This behavior has frequently been cited as reflecting deep state meditation. Analysis of pulse rates showed only small and unsystematic effects. Unfortunately, it was generally not possible to reliably infer the meditation state from the physiological signals.
2.5.2 EEG
Due to technical problems, it was not possible to completely remove the MRI gradient artifacts (Fig. 5 top). After filtering out the remaining frequency components, we could determine a small change in EEG amplitude in different frequency bands.
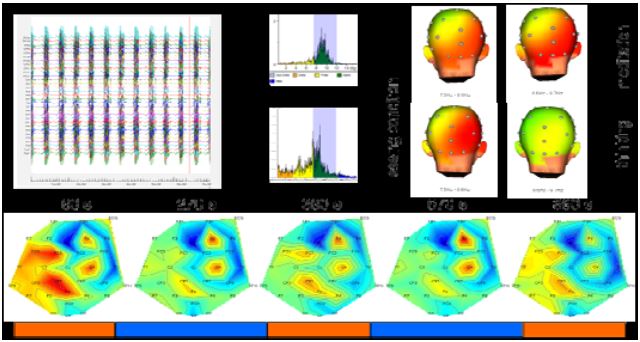
Figure 5: EEG data analysis
While we observed a decrease of beta activity in the meditation period over left parietal and central electrode sites (CZ, CP1, FC), beta activity was increased over the electrodes in right central electrode sites (C4, F4) (Fig. 5 bottom). Preliminary time-frequency analysis of the EEG signals pointed to increased power in the lower alpha bands over frontal and parietal electrode sites during meditation compared to normal thinking conditions in the stimulus switching task.
2.5.3 fMRI
2.5.3.1 Group analysis
We first calculated average group results from all investigations of the meditation condition. Although individual results were quite different, we nevertheless found some interesting activations and deactivations already in our preliminary analysis, which assumed more or less the same activations over the whole meditation periods (Fig. 6). There were higher activations in the visual cortex while performing meditation in the seeing condition, whereas highest activation in the hearing condition was found in the left Heschl’s gyrus. Main activation in the touch condition was located in the left insula, and the cognition task generated additional activations in regions of the parietal lobes.
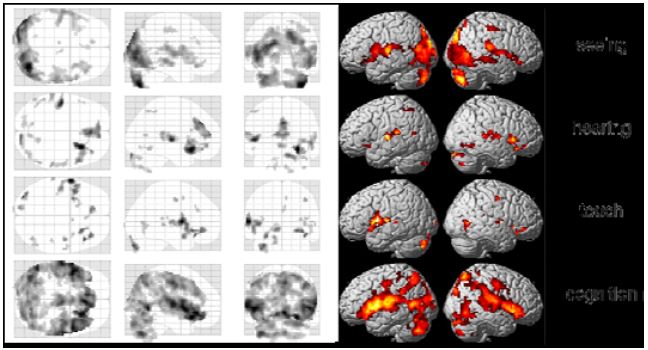
Figure 6: Group results of the four tasks
A more elaborate analysis method allowing transient time courses of the BOLD-signal (the so called independent component analysis, ICA) showed comparable results. We found the following common activations and deactivations in all 4 different meditation types when contrasting meditation with normal thinking (Fig. 7):
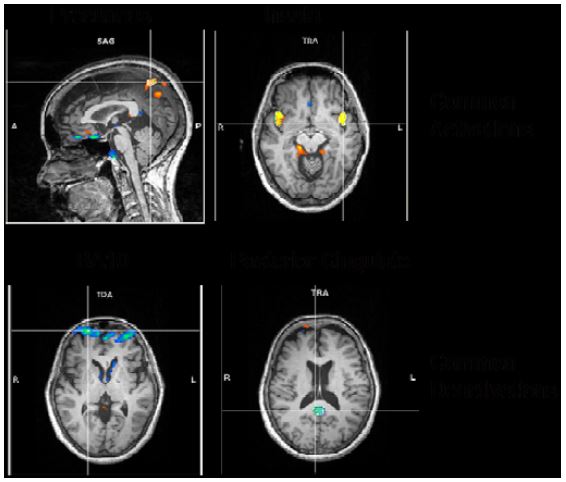
Figure 7: ICA common results
- activations in bilateral precuneus, implicated in self-processing and consciousness (Cavanna 2007),
- activations in the bilateral insula, implicated in interoception (Craig, 2009),
- deactivations in frontopolar region of the brain, namely, BA10, involved in strategic processes including memory retrieval and executive function, and
- deactivations in the posterior cingulate, implicated consistently in the default network of brain function (Raichle, 2001).
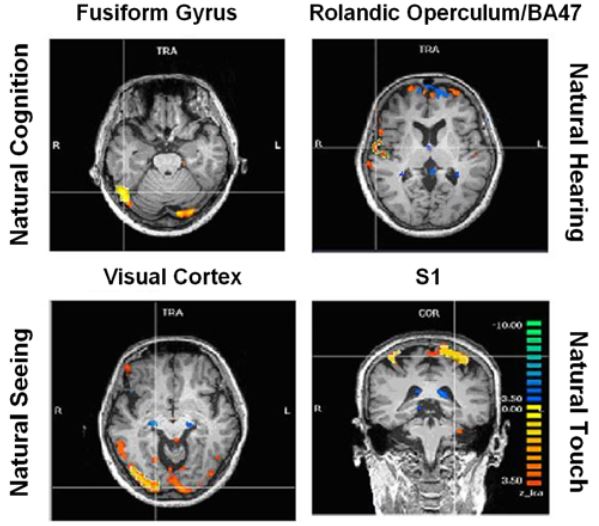
Figure 8: ICA specific results
Activations pertaining to specific meditation types (meditation versus normal thinking contrast) were as follows (Fig. 8):
- enhanced activation of the fusiform gyrus (FFG) during natural cognition meditation condition,
- enhanced activation of the right rolandic operculum and inferior frontal gyrus (BA 47) during the natural hearing meditation condition,
- enhanced activation of the visual cortex during the natural seeing meditation, and
- enhanced activation in the somatosensory cortex during the natural touch meditation condition.
2.5.3.2 Individual analysis from experiments with the Master Thích Thông Triệt
Some experiments were only performed with Master Thích Thông Triệt as he is able to reach deeper and more intense states of mind.
Visual and auditory Naming of animals and tools
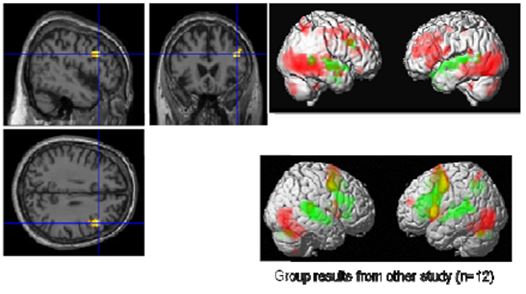
Figure 9: Visual (red) and auditory (green) naming (Master top, group bottom) left: common activation in the right IFG
With the visual and auditory naming task (Fig. 9), we could identify differentially activated regions in visual and auditory association areas and common activations in language areas (Broca’s and Wernicke’s areas). In particular, we found in the visual naming condition activations in the bilateral visual cortex in the ventral stream consisting of middle occipital gyri (BA 18, BA 19), fusiform gyri (BA37), inferior temporal gyri, and middle temporal gyri. These structures are associated with object recognition and form representation. Further, the cerebellum (IX, X) and inferior parietal lobes were activated prominently in the right hemisphere. In contrast to these regions, the superior temporal gyri lighted up only in the auditory naming task. Regions common to both tasks were the triangular part of the right inferior frontal gyrus (IFG) and the left superior temporal gyrus (BA22, BA42). As the Master is right-handed, a right dominance of language processing has low probability but is still possible. In more than 95% of right-handed men and more than 90% of right-handed women, language and speech is sub served by the brain’s left hemisphere, but in left-handed people, the incidence of left-hemisphere language dominance has been reported as 73% and 61% [Knecht 2000]. However, one should exercise caution in concluding that this difference compared to a group of 12 right-handed students is an effect of meditation.
Different levels of thinking
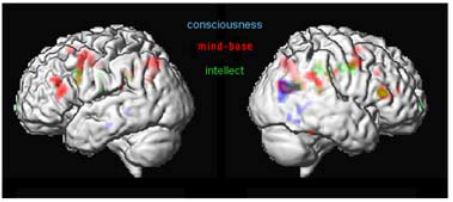
Figure 10: Different levels of thinking
The first analyses of these sessions (Fig. 10) demonstrated common activations in the posterior end of the right middle temporal lobe and the angular gyrus (BA39). This area is implicated in the integration of multimoldal data and interpretation of written words (Damasio 1994). Persinger and colleagues (2001) have shown, that out-of-body experiences and mystic experiences could be triggered, if the region of the temporal lobe is stimulated with transcerebral weak complex magnetic fields. The experimental condition “intellect” showed activations in the dorsolateral superior frontal gyrus. Commonly activated regions with “intellect” and “mind-base” condition activate the right triangular part of the inferior frontal gyrus (Broca’s area, BA 44/45) and the brain regions to the left and right precentral gyrus (premotor cortex, BA6). Brain areas involved in “conscious” thinking were mainly constrained to the temporal and parietal lobe. It is important to note, that the area within BA 44/45 common to “intellect” and “mind-base” thinking is in the same region, but not at the same position as the area in the right frontal lobe that was found to be commonly activated by the visual and auditory naming task.
Different levels of meditation depth (levels of Awareness)
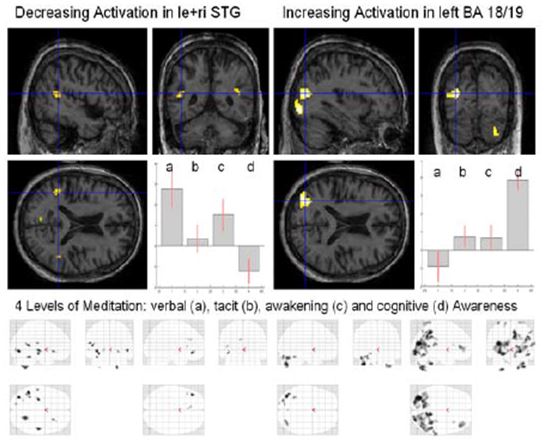
Figure 11: Four levels of Awareness
Analysis of these different meditation levels (Fig. 11) showed a decrease of activation in the left and right superior temporal gyrus (STG) and an increase of activation in the left higher visual areas (BA 18/19), the right inferior frontal gyrus (IFG), the right insula and right cerebellum (Crus1). Inspecting the four different “glass” brains (maximum intensity projection, MIP) (Fig. 10, bottom) one notices a widespread global increase in the occipital lobe for cognitive awareness.
Different Meditation tasks with and without stimulation
In our first experimental protocol (section 2.2.1), external stimulation was held constant to elicit only the differences between normal thinking and meditation. In the currently reported experiment, the design was changed so that the brain state was kept constant (either baseline or meditation), while the external stimulation was switched on and off. For this experiment, we used a design with 11 blocks of 30 seconds resulting in a session length of 5 minutes 30 seconds. Each of the three tasks “seeing”, “hearing” and “touch” were performed two times, first with normal thinking and in the next run with meditation. Comparing the activations induced in these two sessions, we identified differences in processing external stimuli in the different brain states.
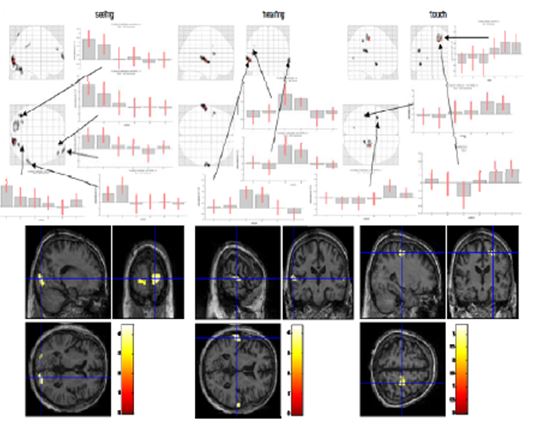
Figure 12: Switching stimulation (seeing, hearing, touch; normal thinking and meditation)
In the “seeing” session (Fig. 12, left), we found activated regions in the primary visual cortex on both sides, the left cerebellum and left fusiform gyrus, the right supramarginal gyrus and inferior parietal lobe (BA40) and the middle and superior frontal gyrus (BA10). In the “hearing” condition (Fig. 12, middle), we identified the main activations in left and right superior temporal gyri (BA22/42) and in the left frontal operculum (BA44/45). In the “touch” condition (Fig. 12, right), we found activations in left insula and rolandic operculum and in right postcentral gyrus, the primary somatosensory area. We generally observed a smaller amplitude in the meditation condition compared to normal thinking in all three primary sensory areas. This can be interpreted as reduced sensitivity to changes of external stimulation in the meditative state. Two explanations are possible, that is either the gain of the external input is decreased or the level of activation is maintained by “filling up” with internal generated activity.
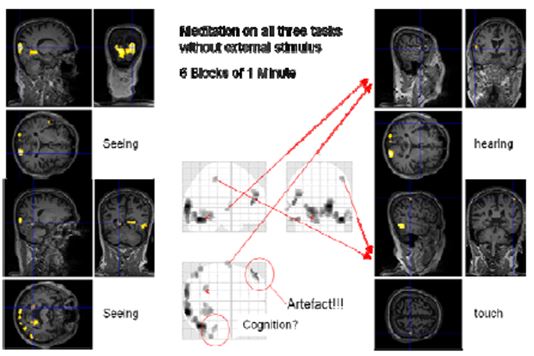
Figure 13: Cognition with block length of one minute
It was not possible to find a comparable design for the “cognition” condition as there was no external input. Therefore, we decided to use a design with 1 minute blocks of normal thinking and meditation without external input and simultaneous concentrating on “seeing”, “hearing”, “touch” and “cognition”. This fast switching between the two states was only possible with a very experienced meditator as Master Thích Thông Triệt. When the blocks were only 30 seconds long, he experienced problems leaving the meditative state. We thus settled for 60 second blocks. In this session, the online evaluation already provided some findings. However, more sophisticated analysis including the consideration of movement parameters resulted in less (false positive) activated regions. Nevertheless, we could identify regions in the visual (occipital lobe, BA17, BA19), auditory (Heschl’s gyrus, BA41) and somatosensory (BA3) areas showing enhanced activation in the meditation state compared to the control state.
3. Conclusions
Our results show that Śūnyatā meditation enhances perception of external stimuli and interoception of internal bodily states, as shown by heightened activations in sensory areas and the insula when compared to the normal, day-to-day thinking state in sessions with long meditation periods (3 minutes) and constant external input. In the sessions with fast changes of external stimuli (30 seconds), the pattern was reversed: brain activation was reduced in the primary sensory areas in the meditation state compared to intellectual thinking. This can be explained by an additional activation in the time periods without external stimulation in the meditation state as was found in the cognitive condition. If this supplementary activation is not purely additive, that is the enhancement of activation with external stimuli is smaller than the inserted activation without external stimuli, it will result in a reduced difference (Fig. 14).
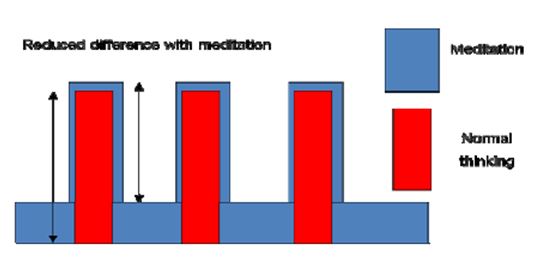
Figure 14: Reduced difference in meditation state
Our findings suggest that Śūnyatā meditation reduces discursive thought as shown by a consistent deactivation of the BA10, a brain region involved in memory retrieval, planning and executive function. It enhances the self-conscious state reflected in the increased activation of precuneus. Being in a meditative state seems to replicate the default state of brain function as shown by the deactivation of the posterior cingulate. The analysis of meditation depth shows decreasing activations in semantic language area (STG) and increasing activations in higher visual areas. Our work supports the view that experienced meditation practitioners are able to produce repeatable and reliable states and verbal reports of meditation, even in the uncomfortable environment of the MR scanner.
Experiments of the kind described here are difficult to perform and still hampered by problems. The findings thus need to be interpreted with caution. Nevertheless, the first results are promising and more evidence needs to be gathered. We plan to expand the study to different levels of experience and other meditation methods to arrive at a framework that allows describing the brain activity in different mental states. The type of investigation performed here requires participants with a strong self-control of mind obtained by many years of training. Observation of mental processes in these participants can help to isolate sub functions of mind and search for the underlying neural substrates.
Acknowledgements
We thank all the participants and supporters of this study from the Śūnyatā Meditation Stuttgart e.V1 (Germany) and monks and nuns from Śūnyatā Meditation Center in Riverside (CA, USA).
1 e. V = eingetragener Verein = Association
In addition, we thank Maike Borutta and Vinod Kumar for assistance with the fMRI measurement; Adrian Furdea and Ander Ramos for conducting EEG measurement; Prof. Dr. Brigitte Stemmer for helpful comments on this manuscript; and last but not least Prof. Dr. Wolfgang Grodd and Prof. Dr. Niels Birbaumer for providing the opportunity to use our time and the equipment to perform this study.
References:
- Anderson, Tenshin Reb: Just sitting . in: Loori , John Daido (ed.) The Art of Just Sitting: Essential Writings on the Zen Practice of Shikantaza. (2002) pp 155-160, Wisdom Publications, Somerville, MA USA.
- Barinaga M: Studying the well trained mind. Science (2003); 302:44-46.
- Cahn BR, Polich J: Meditation States and Traits: EEG, ERP, and Neuroimaging Studies. Psychological Bulletin 132 (2006); 180-211.
- Calhoun VD, Liu J, Adali T: A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage (2009) Mar; 45(1 Suppl):S163- 72. Epub 2008 Nov 13.
- Cavanna AE: The precuneus and consciousness. CNS Spectrum (2007) Jul; 12(7):545-52.
- Craig AD: How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. (2009) Jan; 10(1):59-70.
- Damasio, A. R. & Damasio, H. in Large Scale Neuronal Theories of the Brain (Eds Koch, C. & Davis, J. L.) MIT Press, Cambridge, MA, (1994) 61–74.
- Lutz A, Greischar LL, Rawlings NB, Ricard M, Davidson RJ: Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proc. Natl. Acad. Sci. U. S. A. (2004); 101, 16369–16373.
- Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, Ringelstein EB, Henningsen H. : Handedness and hemispheric language dominance in healthy humans. Brain (2000); 123(12):2512–2518.
- Ogawa S, Lee TM, Nayak AS and Glynn P: Oxygenation sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields, Magn. Reson. Med., 14 (1990), 68-78.
- Persinger MA: The Neuropsychiatry of Paranormal Experiences. J. Neuropsychiatry Clin. Neurosci. 13 (2001) 515-524.
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL: A default mode of brain function. Proc Natl Acad Sci U S A. (2001) Jan 16; 98(2):676-82.
![]()


